
1. Thethi TK et al. Efficacy, safety and cardiovascular outcomes of once-daily oralsemaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020;22(8):1263–77.
2. Candido R. Evoluzione del ruolo dei GLP-1 agonisti recettoriali nel nuovo paradigma del diabete mellito tipo 2. JAMD 2021 Vol. 24/2.
3. Husain M, Bain SC, Jeppesen OK, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020;22(3):442–51.
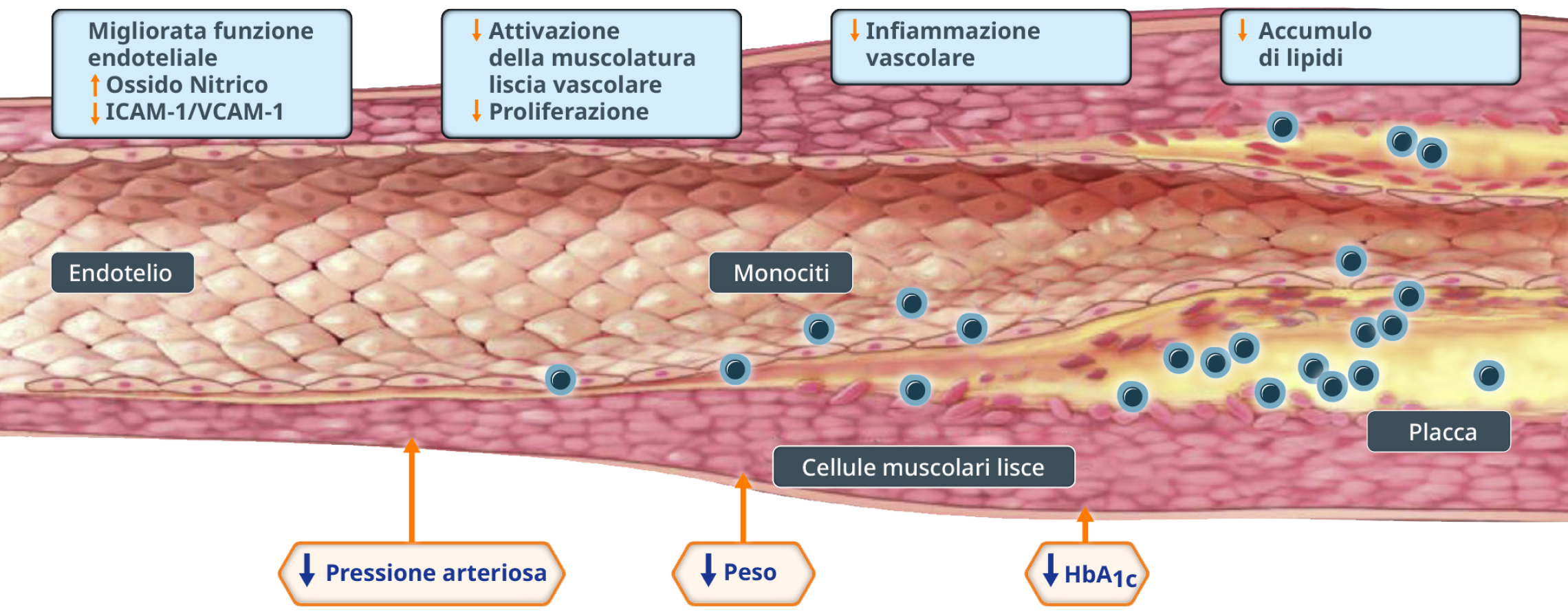
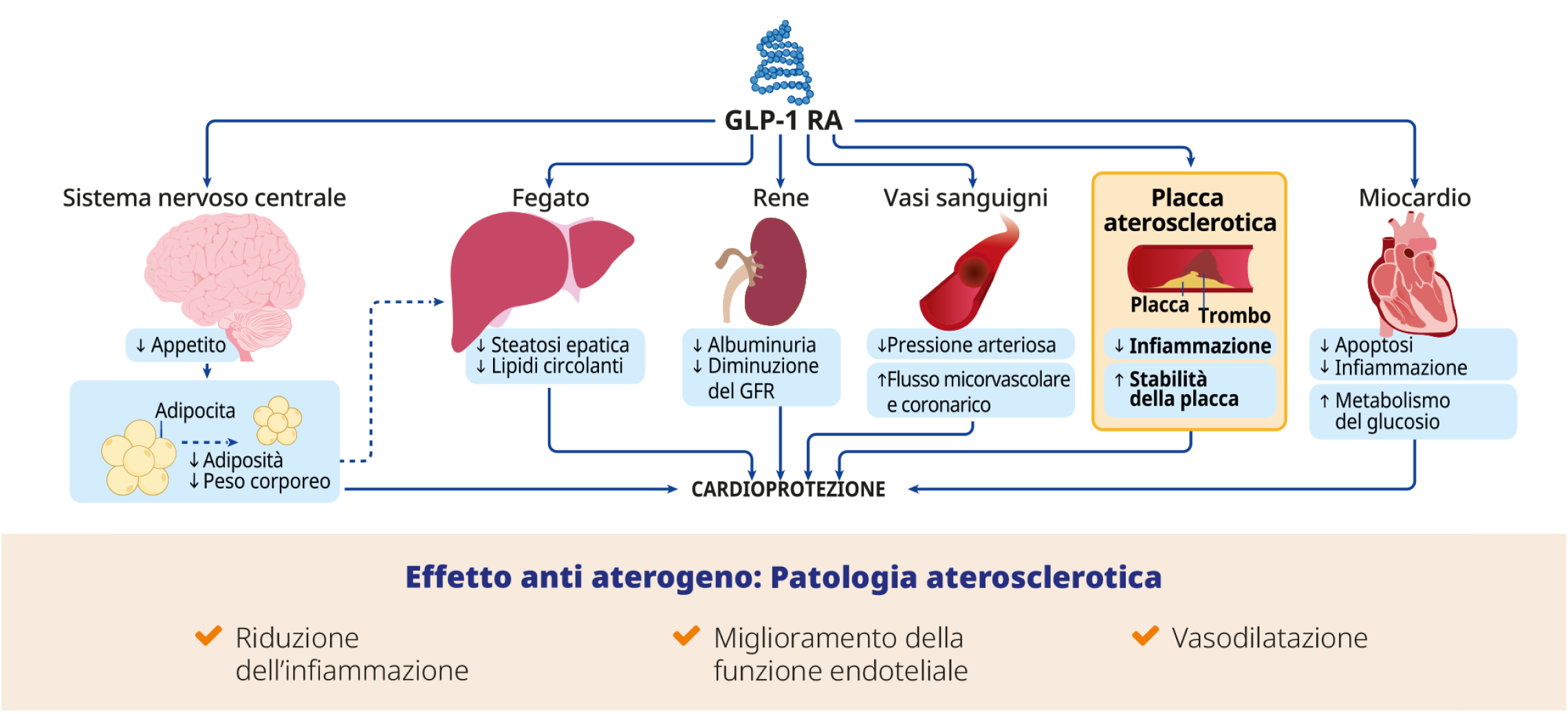
4. Sharma A, Verna S. Mechanisms by Which Glucagon-Like-Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors Reduce Cardiovascular Risk in Adults With Type 2 Diabetes Mellitus. Can J Diabetes 2020; 44: 93-102.
5. Koufakis T et al. A Horse, a Jockey, and a Therapeutic Dilemma: Choosing the Best Option for a Patient with Diabetes and Coronary Artery Disease. American Journal of Cardiovascular Drugs 2022; 22: 357–361.
6. John R. Ussher and Daniel J. Drucker. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Endocr Rev. 2012; 33(2): 187–215.
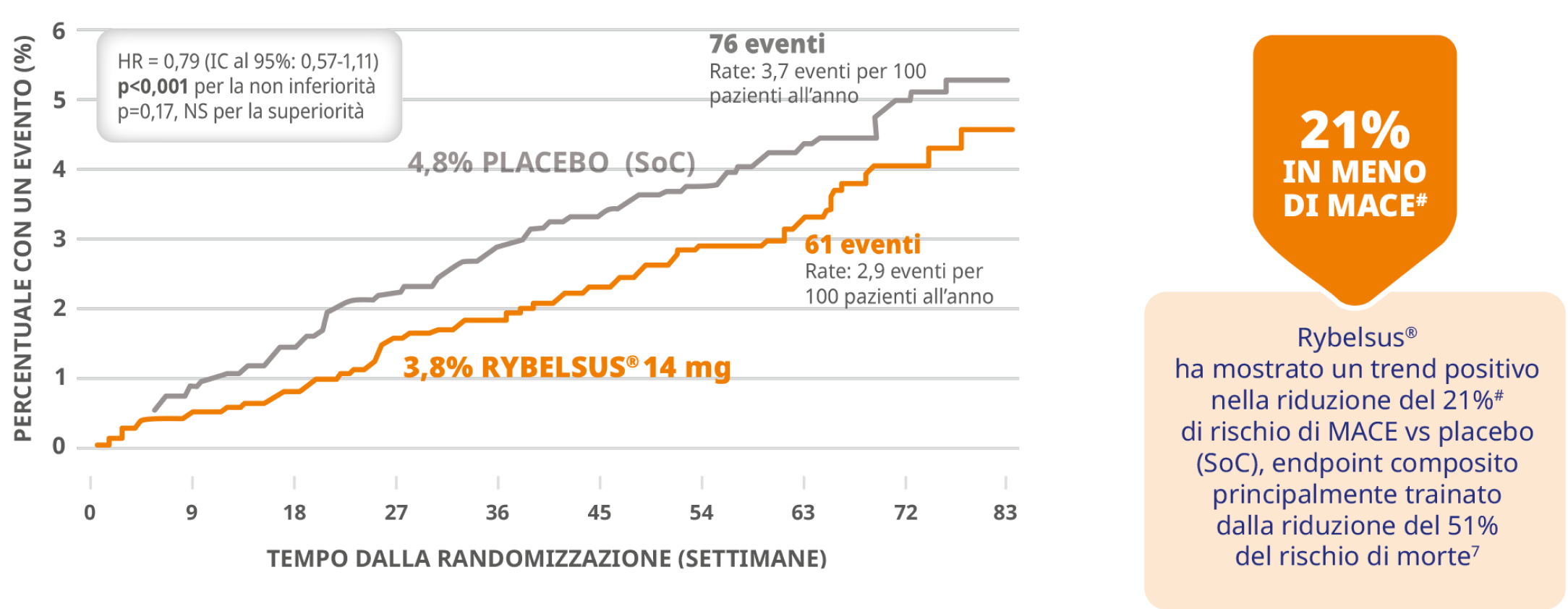
7. Husain M et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2019 Aug 29;381(9):841-851.
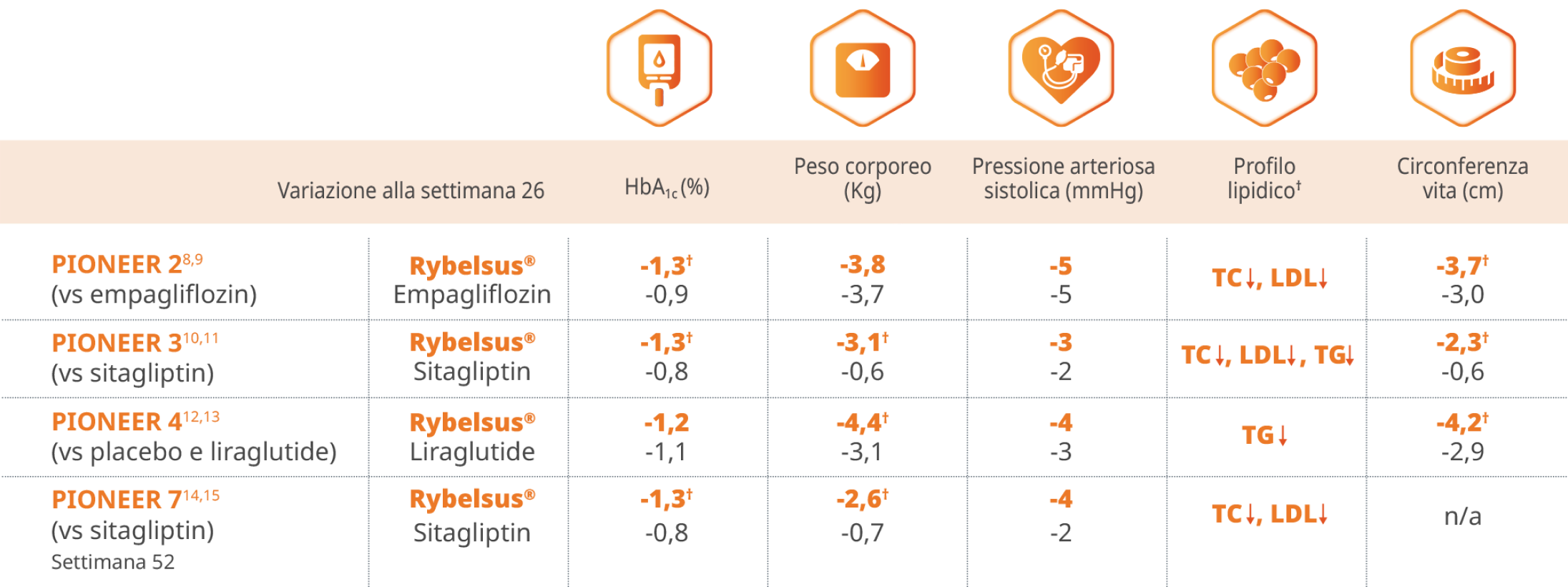
8. Rodbard HW et al. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care 2019;42:2272–2281.
9. Rodbard HW et al. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care 2019;42:2272–2281. Supplementary Material.
10. Rosenstock J et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated
Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA 2019 Apr 16;321(15):1466-1480.
11. Rosenstock J et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA 2019 Apr 16;321(15):1466- 1480. Supplementary Material.
12. Pratley R et al.Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 2019; 394: 39–50.
13. Pratley R et al.Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 2019; 394: 39–50. Supplementary Material.
14. Pieber RT et al. Efficacy and safety of oral semaglutide with flexible dose adjustment
versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol 2019; 7: 528–39.
15. Pieber RT et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol 2019; 7: 528–39. Supplementary Material.
16. EMA Europa, Human medicines highlights, 2020.
17. Rybelsus® Riassunto delle Caratteristiche del Prodotto
Rybelsus® 3 mg - compressa - uso orale - blister (ALU/ALU) - 30 compresse - A.I.C. n. 048719025 /E;
classe di rimborsabilità: A; prezzo al pubblico (IVA inclusa): € 219,09;
Rybelsus® 7 mg - compressa - uso orale - blister (ALU/ALU) - 30 compresse - A.I.C. n. 048719052 /E;
classe di rimborsabilità: A; prezzo al pubblico (IVA inclusa): € 219,09;
Rybelsus® 14 mg - compressa - uso orale - blister (ALU/ALU) - 30 compresse - A.I.C. n. 048719088 /E;
classe di rimborsabilità: A; prezzo al pubblico (IVA inclusa): € 219,09.
Per Rybelsus® RCP comprensivo di classe, prezzo e regime di fornitura clicca qui.
Victoza® A.I.C. 039 365010/E, Prezzo al pubblico € 146,06 (IVA inclusa). Classe A - Medicinale soggetto a prescrizione medica (RR).
Per Victoza® RCP comprensivo di classe, prezzo e regime di fornitura clicca qui.
Medicinale soggetto a prescrizione medica (RR).
Materiale destinato unicamente agli operatori sanitari. Vietata la distribuzione o l’esposizione al pubblico.
Rybelsus® è un marchio registrato di proprietà della Novo Nordisk A/S
Materiale promozionale depositato presso AIFA in data 07/02/2024 Codice Deposito Aziendale IT23RYB00138




