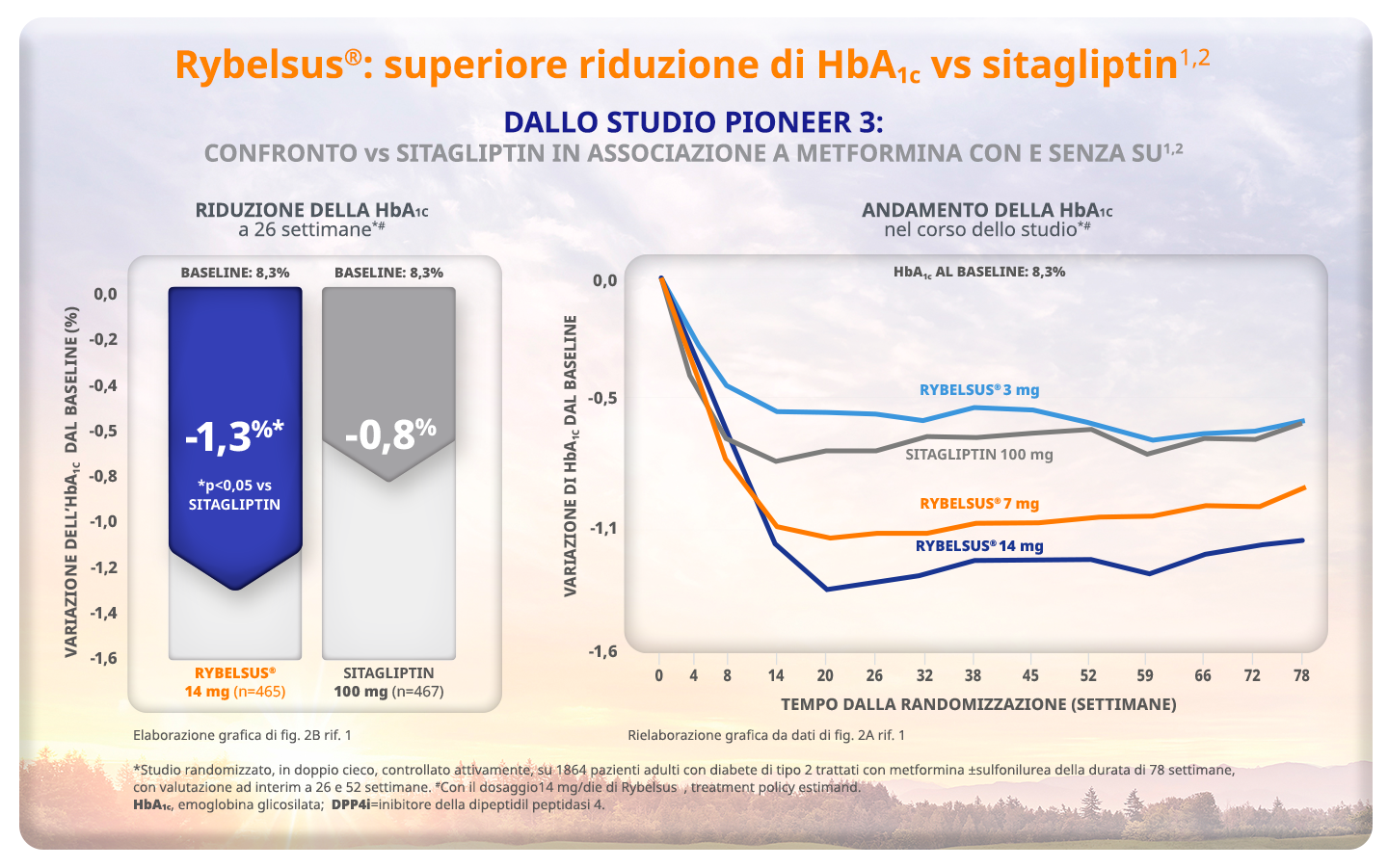
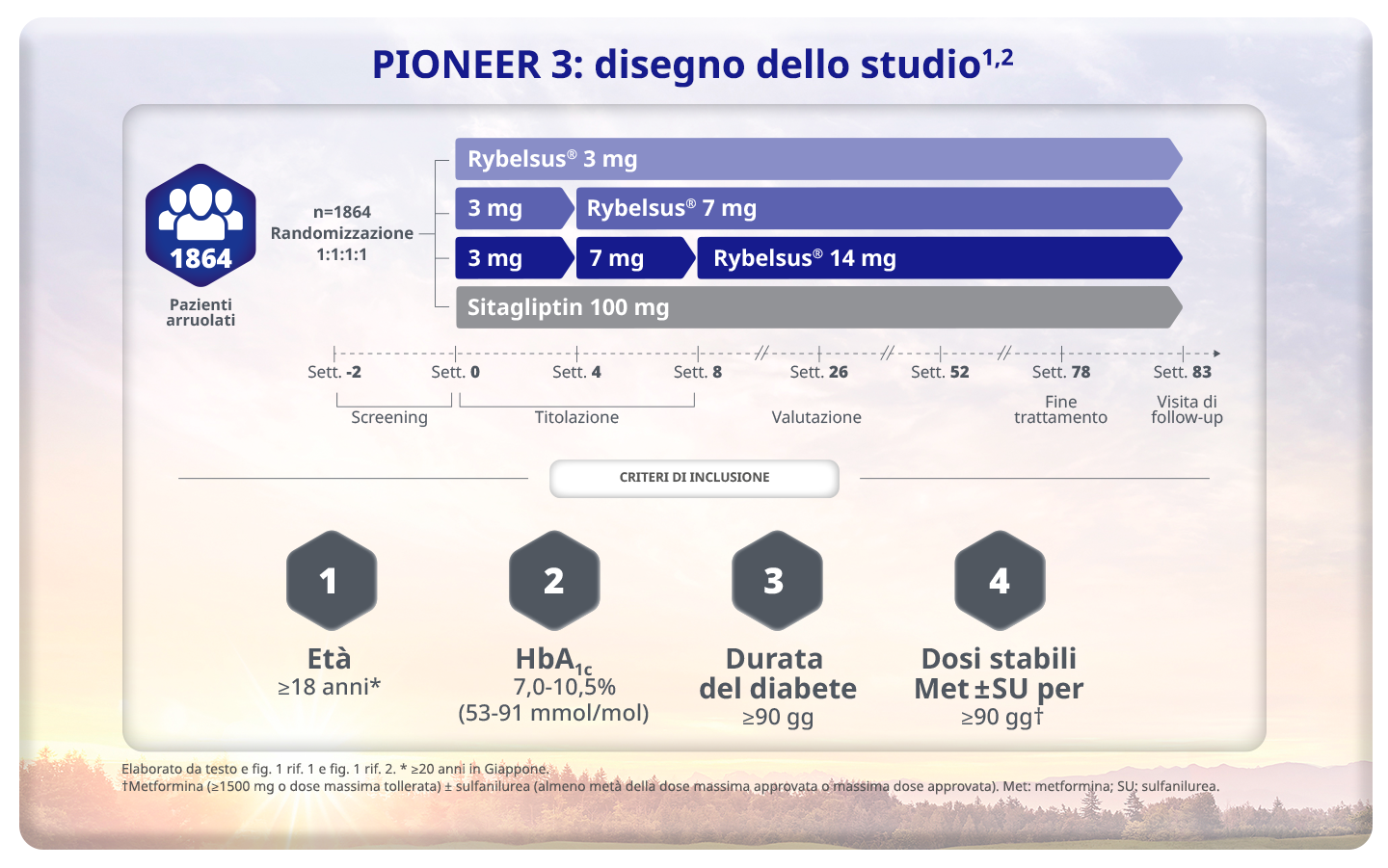
Rosenstock J et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA 2019 Apr 16;321(15):1466-1480
Rosenstock J et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA 2019 Apr 16;321(15):1466-1480. Supplementary Material
Thethi TK et al. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: The PIONEER programme. Diabetes Obes Metab 2020;22:1263–1277
Rodbard HW et al. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care 2019;42:2272–2281
Rodbard HW et al. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care 2019;42:2272–2281. Supplementary Material
Nuhoho S et al. Orally Administered Semaglutide Versus GLP-1 Ras in Patients with Type 2 Diabetes Previously Receiving 1–2 Oral Antidiabetics: Systematic Review and Network Meta-Analysis. Diabetes Ther (2019) 10:2183–2199
EMA Europa, Rybelsus RCP, 2021.
Rybelsus® 3 mg - compressa - uso orale - blister (ALU/ALU) - 30 compresse - A.I.C. n. 048719025 /E; classe di rimborsabilità: A; prezzo al pubblico (IVA inclusa): € 219,09;
Rybelsus® 7 mg - compressa - uso orale - blister (ALU/ALU) - 30 compresse - A.I.C. n. 048719052 /E; classe di rimborsabilità: A; prezzo al pubblico (IVA inclusa): € 219,09;
Rybelsus® 14 mg - compressa - uso orale - blister (ALU/ALU) - 30 compresse - A.I.C. n. 048719088 /E; classe di rimborsabilità: A;prezzo al pubblico (IVA inclusa): € 219,09.
Per Rybelsus® RCP comprensivo di classe, prezzo e regime di fornitura clicca qui.
Medicinale soggetto a prescrizione medica (RR).
Materiale destinato unicamente agli operatori sanitari. Vietata la distribuzione o l’esposizione al pubblico.
Rybelsus® è un marchio registrato di proprietà della Novo Nordisk A/S
Materiale promozionale depositato presso AIFA in data 10/05/2022 IT21RYB00025